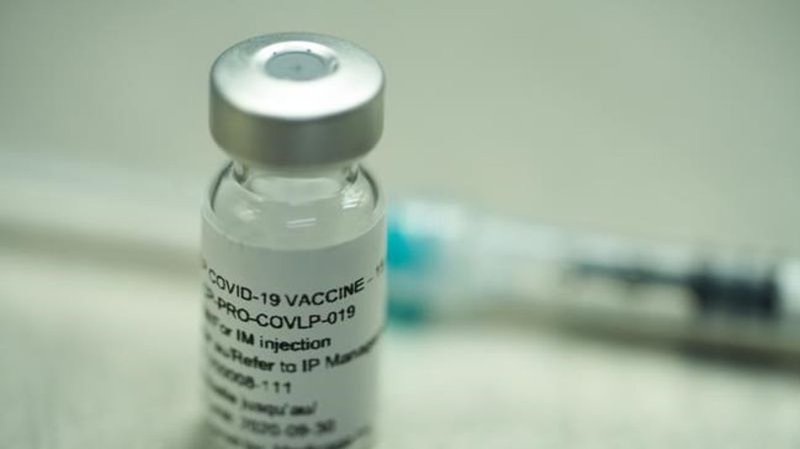
WHO rejects Canadian-made COVID-19 vaccine from Medicago, with ties to big tobacco
OTTAWA — The World Health Organization has rejected Medicago’s made-in-Canada COVID-19 vaccine, restricting the federal government’s ability to donate those doses to countries in need.
The federal government signed an agreement with Medicago in 2020 to buy 20 million doses once the vaccine was approved by Health Canada, with the option to purchase 56 million more.
Health Canada authorized Medicago’s two-dose Covifenz vaccine in February for adults 18 to 64.
In clinical trials it was more than 70 per cent effective at preventing COVID-19 infections and 100 per cent effective against severe illness, before the Omicron wave.


